Research Articles
Automated Synthesis Platforms: A Cost-Benefit Analysis for Accelerating Drug Discovery
This article provides a comprehensive cost-benefit analysis of automated synthesis platforms for researchers, scientists, and drug development professionals.
Batch vs Flow Automated Synthesis: A Strategic Comparison for Modern Drug Development
This article provides a comprehensive comparison of batch and flow automated synthesis platforms for researchers, scientists, and drug development professionals.
UPLC-MS vs. NMR in Automated Workflows: A Strategic Guide to Validation, Integration, and Application
This article provides a comprehensive comparison of Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) and Nuclear Magnetic Resonance (NMR) spectroscopy within the context of modern, automated laboratories.
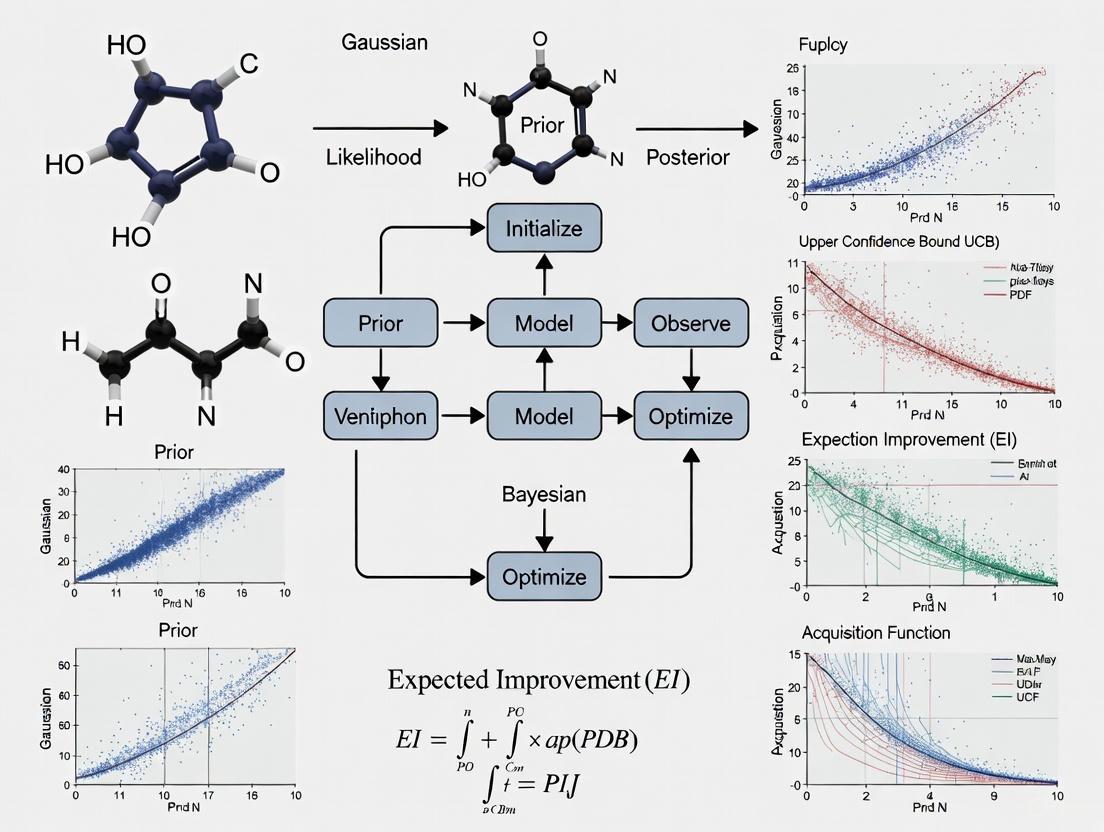
Bayesian Optimization in Chemical Synthesis: Accelerating Drug Discovery with AI
This article explores the transformative role of Bayesian Optimization (BO) in efficiently identifying optimal chemical reaction conditions, a critical and resource-intensive challenge in pharmaceutical development.
Miniaturized Reactions: Strategies for Reducing Material Consumption in Biomedical Research
This article provides a comprehensive guide for researchers and drug development professionals on leveraging reaction miniaturization to drastically reduce material consumption.
Automating Air-Sensitive Chemistry: Strategies for Safe, Efficient, and Scalable Synthesis
This article provides a comprehensive guide for researchers and drug development professionals on automating air-sensitive chemical synthesis.
Error Handling in Autonomous Synthesis Platforms: From Failure Management to Robust Discovery
This article provides a comprehensive analysis of error handling strategies for autonomous synthesis platforms used in chemical and materials discovery.
Overcoming Temperature Control Challenges in Automated Reactors: From Fundamentals to AI-Driven Solutions
This article provides a comprehensive analysis of temperature control challenges in automated reactors, tailored for researchers, scientists, and drug development professionals.
Overcoming Solvent Compatibility Challenges in Automated Liquid Handling: A Guide for Robust and Reproducible Science
Solvent compatibility remains a critical bottleneck in automated liquid handling, directly impacting data integrity, assay reproducibility, and operational efficiency in drug discovery and clinical research.
Machine Learning in Chemical Synthesis: A Practical Guide to Optimizing Reaction Yields for Drug Development
This article explores the transformative role of machine learning (ML) in optimizing chemical reaction yields, with a specific focus on applications in pharmaceutical development.